Plant Growth and Cultivation Research Plant Culture System Program (3)
 Plant culture is one of the most important routine basic experiments in biological laboratories. In previous studies, only the culture system was required to enable the seeds to germinate and basically satisfy the growth of the plants. However, in the study of truly rigorous plant physiology and ecology, traditional incubators are far from being able to meet the requirements for various reasons.
Plant culture is one of the most important routine basic experiments in biological laboratories. In previous studies, only the culture system was required to enable the seeds to germinate and basically satisfy the growth of the plants. However, in the study of truly rigorous plant physiology and ecology, traditional incubators are far from being able to meet the requirements for various reasons.
This article will introduce a series of scientific research plant culture programs based on LED light source, including SL3500 plant culture LED light source, FytoScope plant growth box and so on. These culture programs and instruments were directly designed by European plant physiology scientists to be able to conduct accurate scientific research experiments.
Published literature and application cases
1. Effects of leaf and plant age on photosynthetic capacity in Arabidopsis Bielczynski LW, et. al, 2017, Leaf and plant age affects photosynthetic performance and photoprotective capacity. Plant Physiology, DOI: https://doi.org/10.1104/pp .17.00904
In order to study the effects of Arabidopsis leaves and plant age on photosynthesis ability, Bielczynski used FytoScope growth chamber to simulate long-term high-light adaptation culture process with the highest light intensity of 1800 μmol (photons)/m2.s. At the same time, the FluorCam chlorophyll fluorescence imaging system was used to measure the chlorophyll fluorescence of Arabidopsis plants and leaves at different growth stages (Fig. 10), and it was found that the steady photosynthetic capacity was gradually increased during the growth of Arabidopsis. Under high light stress, the adaptability of old leaves is lower than that of young leaves.
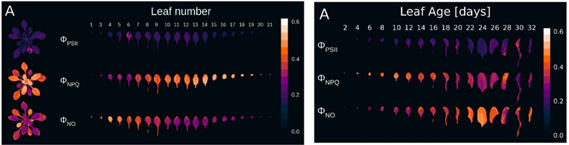
Figure 10. Chlorophyll fluorescence imaging of different ages and leaves of different leaves 2. Arabidopsis genotype and phenotype monitoring Pavicic M, et. al, 2017, Genomic and Phenomic Screens for Flower Related RING Type Ubiquitin E3 Ligases in Arabidopsis. Frontiers in Plant Science, doi: 10.3389/fpls.2017.00416
Flowering regulation is the combination of endogenous signals and environmental signals to promote flower development. Therefore, in the study of related gene expression, environmental conditions need to be strictly controlled, and correlation analysis is performed in combination with Phenotyping phenotype imaging. Pavicic of the University of Helsinki in Finland studied the Arabidopsis ubiquitin E3 linker enzyme by regulating the strict circadian rhythm culture process through the FytoScope growth chamber, combined with the PlantScreen high-throughput phenotypic imaging analysis system (Fig. 11, Fig. 12). The rosette development phenotype of mustard was analyzed. For this study, they developed a specialized experimental procedure through these two systems to monitor high-throughput phenotypes of development, morphology, and flowering in nearly 1,000 Arabidopsis thaliana in one experimental cycle.

Figure 11. PlantScreen Plant Phenotype Imaging Analysis System Automatic Transfer Edition from Helsinki University, Finland + FytoScope Walk-in Large Growth Room
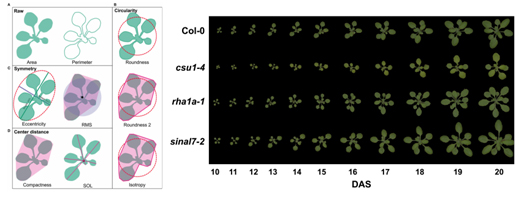
Figure 12. Morphological parameters and growth dynamics of Arabidopsis rosettes 3. ABA-induced regulation of stomatal closure in Arabidopsis Eisenach C, et. al, 2017, ABA-Induced Stomatal Closure Involves ALMT4, a PhosphorylationDependent Vacuolar Anion Channel of Arabidopsis. Plant Cell, doi:10.1105/tpc.17.00452
The opening and closing of stomatal pores is closely related to environmental factors such as light, temperature and humidity. Therefore, in the study of related genes and regulation, these environmental factors must be precisely controlled. In this study, the FytoScope growth chamber was used to accurately simulate the light-dark alternating culture cycle to measure the response of stomata to ABA under different light and dark conditions (Fig. 13).
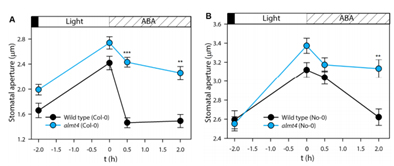
Figure 13. Response of stomatal to ABA under light and dark conditions 4. Coastal plant response to climate change
Duarte used FytoScope to simulate diurnal variation to study the physiological changes of C3 plant Halimioneportulacoides and C4 plant Spartinamaritima under different dissolved CO2 conditions, and to explore the response of salt marsh plants to climate change. On the one hand, FytoScope can regulate temperature, light and day and night; on the other hand, FytoScope can also precisely control CO2 concentration (Duarte, 2014).
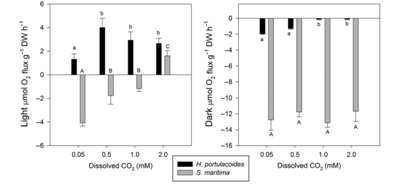
Figure 14. Production and consumption of oxygen in two plants under different CO2 and light conditions Duarte uses a dissolved oxygen meter (RF-O2 fluorescent fiber oxygen measurement technology) to measure the rate of oxygen evolution of the two plants under different CO2 and light conditions (Figure 8); the OJIP curve is also measured by the MP100 chlorophyll fluorescence monitor in FytoScope. More than ten fluorescence parameters, such as Fv, QY, ABS/CS, TR0/CS, and ET0/CS, were used to analyze the effects on photosynthetic systems (Figure 15).
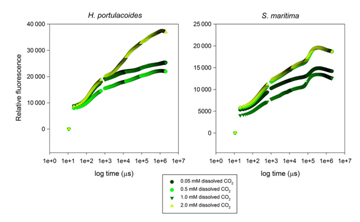
Figure 15. OJIP kinetic curves of two plants under different CO2 conditions Finally, Duarte believes that salt marshes play an important role in the compensatory effects of climate change through the oxidation of water bodies and the acidification buffering of excess CO2.
Effects of heavy metal stress on seed germination and photosynthetic physiology
Santos used FytoScope to study the hyperaccumulation of Zn in the genus Juncusacutus (Santos, 2014). J. acutus was cultured by setting a series of different concentrations of Zn stress gradient, and growth indexes such as germination rate and dry weight were measured (Fig. 16). The FP100 chlorophyll fluorescence meter was used to analyze the damage of Zn to its photosynthetic system (Fig. 17).

Figure 16. Germination of J. acutus in different concentrations of Zn
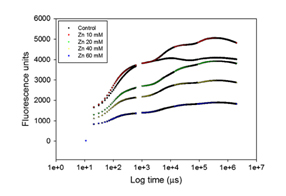
Figure 17. OJIP kinetics curves of J. acutus in different concentrations of Zn The final conclusion of Santos is to show the high tolerance of J. acutus to high concentrations of Zn, while resisting the excessive oxide accumulation caused by Zn on the chloroplast membrane. Therefore, J. acutus can be used for ecological restoration of heavy metal pollution on land and water.
6. Effects of high light stress on algae
Domingues studied the response mechanism of diatom Phaeodactylumtricornutum to photooxidative stress caused by high light (Domingues, 2012). It was found that diatoms after low light adaptation (40 μmol (photons)/m2.s) were subjected to high light (1250 μmol (photons)/m 2 .s) irradiation, resulting in a rapid response of non-photochemical quenching (NPQ) ( FIG. 18 ). Moreover, high light has the same effect on lincomycin as the maximum quantum yield (Fv/Fm), ie a significant reduction in the active PSII reaction center.
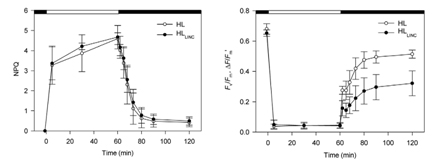
Figure 18. Changes in P. tricornutum NPQ and Fv/Fm Domingues believes that P. tricornutum will distribute more total protein to the photoinhibition target protein D1 under high light and activate the D1 repair cycle to limit photoinhibition.
references: 1. Yan Xinfang et al., 2009, Application of LED light source in plant tissue culture, Chinese Agricultural Science Bulletin, 12:42-45
2. Jaillais Y, et. al, 2010, Unraveling the paradoxes of plant hormone signaling integration, Nature Structural & Molecular Biology, 17:642–645
3. Bielczynski LW, et. al, 2017, Leaf and plant age affects photosynthetic performance and photoprotective capacity. Plant Physiology, DOI: https://doi.org/10.1104/pp.17.00904
4. Pavicic M, et. al, 2017, Genomic and Phenomic Screens for Flower Related RING Type Ubiquitin E3 Ligases in Arabidopsis. Frontiers in Plant Science, doi: 10.3389/fpls.2017.00416
5. Eisenach C, et. al, 2017, ABA-Induced Stomatal Closure Involves ALMT4, a PhosphorylationDependent Vacuolar Anion Channel of Arabidopsis. Plant Cell, doi:10.1105/tpc.17.00452
6. Duarte B, et. al, 2014, Light–dark O2 dynamics in submerged leaves of C3 and C4 halophytes under increased dissolved CO2: clues for saltmarsh response to climate change, AoB PLANTS, doi: 10.1093/aobpla/plu067
7. Santos D, et. al, 2014, Unveiling Zn hyperaccumulation in Juncusacutus: Implications on the electronic energy fluxes and on oxidative stress with emphasis on non-functional Zn-chlorophylls, Journal of Photochemistry and Photobiology B: Biology, 140:228- 239
8. Domingues N, et. al, 2012, Response of the diatom Phaeodactylumtricornutum to photooxidative stress resulting from high light exposure, PLoS one, 7(6): e3816
Related Links:
Scientific research plant culture program based on LED light source (1)
Scientific research plant culture program based on LED light source (2)

 Plant culture is one of the most important routine basic experiments in biological laboratories. In previous studies, only the culture system was required to enable the seeds to germinate and basically satisfy the growth of the plants. However, in the study of truly rigorous plant physiology and ecology, traditional incubators are far from being able to meet the requirements for various reasons.
Plant culture is one of the most important routine basic experiments in biological laboratories. In previous studies, only the culture system was required to enable the seeds to germinate and basically satisfy the growth of the plants. However, in the study of truly rigorous plant physiology and ecology, traditional incubators are far from being able to meet the requirements for various reasons. 









